Hydrocarbon Gas Chromatography Analysis In Oil & Gas article is one of 5 important articles about Mud logging unit equipment. These articles explain in detail the equipment used in standard mud logging units.
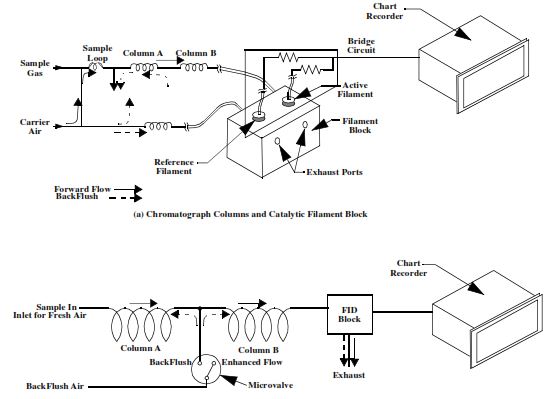
A chromatograph separates and analyzes hydrocarbons in the ditch gas sample to determine how much of each hydrocarbon is contained in the sample. There are two common types of chromatography: the catalytic detector and the flame ionization detector (FID). Each separates and records the gases in a similar manner; the difference between the two is the way in which the various gases are detected once separation has occurred.
Catalytic Gas Chromatograph For Hydrocarons.
The catalytic chromatograph separates & analyses the hydrocarbons by passing the sample through two aluminum columns containing a compound of hexadecane and firebrick (diatomaceous earth). A predetermined quantity of the sample is cycled through the columns at 5-minute intervals. The principle of mud logging gas chromatography being, that when a gas sample is forced through a medium having a large surface area, the process of adsorption causes different compounds to move at different rates based on their molecular size.

Lighter hydrocarbons pass through the columns first, followed by the heavier molecules. The order in which these hydrocarbons arrive at the detector is methane (C1), ethane (C2), propane (C3), isobutane (iC4), and normal butane (nC4). The columns are held at a constant temperature between 100 to 150o F inside the oven to ensure a constant flow rate through the columns. The hydrocarbons flowing through the filament block catalyze the active platinum filament.
When the hydrocarbon that is to be tested enters the detection chamber, the carrier air and the hydrocarbon combine on the filament. The filament remains unchanged, but the catalysis causes the filament to heat in proportion to the hydrocarbon concentration in the sample. When catalysis occurs, both the current through, and resistance of, the filament change, and the output of the bridge varies. The output of the bridge then goes to the recorder.
If higher than butane analyses is required (e.g. pentanes (C5)), the chromatograph can be set to HOLD and the cycle is extended beyond the normal 5 minutes.
There are a few disadvantages to the catalytic chromatograph:
- The theoretical upper limit of sensitivity of the hot-wire filament for methane is 9.5 percent. At higher concentrations, reversals occur due to insufficient oxygen being available for complete combustion, and the excess methane cools the filament.
- It has a negative response to carbon dioxide.
- It is affected by large amounts of nitrogen and suffers thermal drift during temperature changes.
Mud Logging Gas Chromatography – FID
The FID gas chromatography, using the same principles, utilizes two stainless steel columns, packed with large surface area polymer beads, to separate and analyse the hydrocarbons gases. Once separation has occurred, the individual hydrocarbons go to a circular chamber inside an aluminum block for detection. This chamber (the FID chamber) completely encloses a hydrogen flame that is not affected by logging unit pressure or by normal amounts of carbon dioxide and nitrogen.
Hydrocarbons enter the high potential hydrogen flame and are ionized. The detector response will be essentially proportional to the carbon content of the molecule and will depend on the quantity of gas entering the flame per unit of time. The response to ion flow is sent to a high-gain amplifier, then to a chart recorder and recording integrator.
The FID has a greater dynamic range and is more linear to higher gas concentrations than the catalytic chromatograph. It is also less likely to be affected by temperature change.
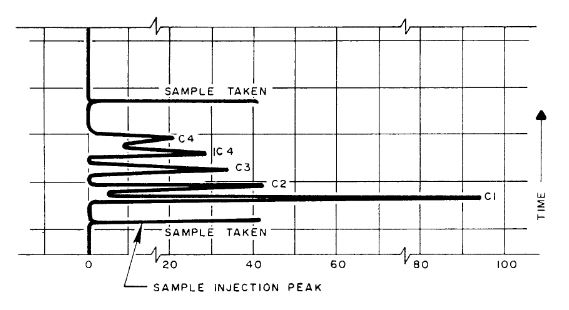
The Chromatogram
The recording (or “signature”) of the gas-air mixture is termed a chromatogram. The sensitivity of the detector to each gas is established on a regular basis by passing a calibrated sample through the columns. This calibration mixture contains known concentrations of methane through pentane.
Mud Logging Gas Chromatography -Related Scientific Papers
Gas logging
Abstract:
Presentation to illustrate how gas is detected and measured during drilling using mud logging services.
Concepts and Methods for the Prediction of Reservoir Hydrocarbon Type Using Ratios of Gas Chromatography C1-C5 Gases
Abstract:
The presentation will help provide greater understanding of the application of gas ratio analyses for the purposes of predicting the hydrocarbon type from which the gases were liberated during drilling. Using the various ratios described and contained in this presentation, it becomes possible to predict and interpret the hydrocarbon source types (not to be confused with the source rock). This is possible based on the premise that rock cuttings from any particular formation “produce” the gases, or the hydrocarbon vapors they contain, into the drilling mud. These same gases are detectable at the surface with the use of Gas Chromatography. It is reasonable to assume that the same formation, if completed, would produce gases of a similar composition. The use of ratios becomes a help in “fingerprinting” the source hydrocarbons. The presentation begins with an overview of basic concepts, then presents various analytical tools and techniques, discusses data applications and concludes with examples of how the ratios are integrated into and enhance reservoir description using the techniques presented