OBM oil-based mud requires special chemical additives to ensure that the emulsion is extremely stable and can withstand conditions of high temperature and contaminants. Oil mud products must be dispersible in the external oil phase. We believe you might also like this article (Baroid Drilling fluids).
The major OBM oil-based mud chemical additives that will be discussed in this article are:
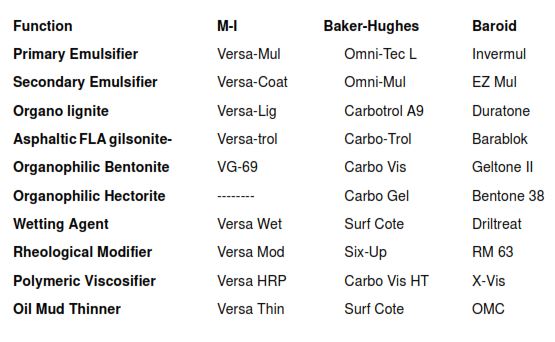
- Primary Emulsifier
- Secondary emulsifiers
- Organophilic lignites
- Organophilic gellants
- Wetting agents
- Polymeric viscosifiers
- Rheological modifiers
- Weighting Agents
Primary Emulsifier
calcium soaps are the primary emulsifier in oil muds. These are made in the mud by the reaction of lime and long-chain (C-16 to C-22) fatty acids. Soap emulsions are very strong emulsifying agents but take some reaction time before the emulsion is actually formed.
Wetting agents prevent solids from becoming water-wet while the emulsion is forming. Emulsifiers surround the water droplets and prevent their coalescence. Invert emulsion drilling fluids are three-phase systems: a continuous phase of oil (diesel, mineral oil, or alternative oil) and two discontinuous solids and emulsified water phases. Since the oil is the continuous phase, the oil surrounds the water droplets and the solid particles. The water will not dissolve in the oil but remain suspended as small droplets. Putting mechanical energy into the system creates such emulsions.
The energy breaks down the emulsified phase into small droplets. However, these drops only remain stable for a short time before they coalesce and form larger droplets. Over time, the water and oil will separate, with water migrating to the bottom and oil to the top. Like those found in properly formulated oil-based invert emulsion muds, stable emulsions should not exhibit phase separation. Surfactants (emulsifiers) are used to stabilize these emulsions. Solids, particularly clay solids, also function as emulsifiers for water-in-oil. Muds that are stabilized with emulsifiers and/or clays will be oil continuous but may exhibit settling of solids if the viscosity is too low.
A surfactant is a molecule with two chemical groups having different natures joined to form a tadpole-like structure. The “tail” is a fatty oil-compatible group containing carbon and hydrogen atoms, while the “head” is a polar, water-compatible group. The polar group may be an alcohol, amine, amide, acid, or salt of an acid. Changing the combination of oil-compatible and water-compatible groups makes it possible to create many surfactants. As two water droplets come together, they repel each other rather than colliding and combining to form one larger droplet. The type of surfactant and the amount of mechanical energy used to mix the mud determine the size of the water droplets. With increased agitation, smaller droplets form, and a tighter emulsion is produced. Smaller water droplets result in a larger surface area and a greater area of oil/water contact.
Secondary emulsifiers Additives For Oil-Based Drilling Mud
The secondary emulsifiers are very powerful oil-wetting chemicals. Generally, these products do not form emulsions as well as the primary emulsifiers, but the oil-wet solids before the emulsion is formed. Used to readily emulsify any water intrusions quickly. Typically, these additives are polyamides or imidazolines.
Organophilic lignites
The organophilic lignites are used as high-temperature fluid loss additives. They also aid in the emulsification of water, especially at high temperatures. Lignite is treated with an amine to make it oil-dispersible. It controls fluid loss by plugging and can be used at high concentrations without causing excessive viscosities (20-lb/bbl +/-). Oil-based mud Asphaltic fluid loss additives – generally consist of Gilsonite or asphalt derivatives. Gilsonite has high-temperature stability (400°F), whereas asphalt is not as temperature stable (350°F). High concentrations of Gilsonite can cause excessive viscosity and gelation of the mud. The treatment level will not usually exceed 8 lb/bbl.
Organophilic Gellants Chemicals For Oil-Based Drilling Mud
Invert emulsions derive limited viscosity from the base oil and submicron-sized emulsified water, but this viscosity will be Newtonian in character. The major non-Newtonian viscosity is derived from organophilic clays (organoclays). These are bentonites in which the inorganic exchangeable cations, such as sodium, calcium, and magnesium, have been displaced by fatty quaternary amines. These viscosity builders are made from bentonite, hectorite, or attapulgite treated with an amine to make them oil-dispersible. Bentonite is most commonly used and is compatible with diesel and mineral oils up to 350°F. Hectorite-based clay should be used for temperatures above 350°F, especially in mineral oil formulations. Organophilic attapulgite improves the suspension properties of well packer fluids without appreciably increasing the viscosity.
The separation of the clay platelets can be helped by adding chemical species that will adsorb between the inter-laminar sites and begin to weaken the bonding forces between the sheets, which can help the separation of the clay platelets. These chemicals are called polar activators. They also act on uncoated sites. The polar activator most often used is water. Freshwater is more effective than calcium chloride brines, so the order of salt addition can be important. This is why adding water with the organophilic clay will enhance the viscosity and dispersion. These materials appear to function by adsorbing onto organophilic clay particles and enhancing interaction among them. Polar activators enable the formation of a network or structure that increases viscosity. The network’s nature is considered different than the viscosity-building structure that forms among clay particles in water-based muds. Although the water is an excellent polar activator, its effect on viscosity is somewhat diluted by the emulsifiers always present in inverted muds.
Aromatics are present naturally in diesel and are responsible for the ease with which organophilic clays (OPC) yield in diesel oil. With the advent of low-toxicity mineral oils as diesel replacements, yielding OPCs was made more difficult. That problem was originally solved by reformulating the OPCs. More recently, similar results have been obtained by adjusting the emulsifier packages. The new generation of oils, namely the esters and ethers, exhibit several-fold higher viscosities than the mineral oils. However, they approach the mineral oils insofar as their inability to yield OPCs.
Wetting Agents Additives For Oil-Based Drilling Mud
The viscous properties of the drilling fluid may be derived from other solids, such as drilled solids and weighting material. These solids will adsorb the surfactants and will likely be mainly oil-wet. However, if insufficient surfactants are present, the solids can contact each other through a layer of water, generating high viscosities. Water-wet solids, particularly barite, can cause viscosity problems at the solids removal equipment, ultimately resulting in the removal of the barite. The problem can be avoided by maintaining the correct level of emulsifier and can be cured by the addition of surfactants called oil-wetting agents. These are supplemental additives to quickly and effectively oil-wet solids that become water-wet. Drill solids and weighting agents will naturally water-wet, and the wetting agents will strip off the water and replace it with an oil layer.
Polymeric viscosifiers
The Polymeric viscosifier additives that increase the viscosity of oil muds in the presence of organophilic bentonite, especially when high temperatures reduce the organophilic bentonite performance; they work up to 400°F. High molecular weight sulfonated polystyrene becomes effective only when the temperature exceeds 250°F.
Rheological Modifiers Chemicals For Oil-Based Drilling Mud
Generally, oil-based muds behave as pseudoplastic fluids. They thin with increasing shear-like clay-containing water-based muds. However, absolute viscosities tend to be considerably lower in oil-based muds. A consequence is that oil-based muds generally do not suspend solids, such as weighting materials and drill cuttings, as well as water-based muds with similar viscosity. This property is particularly important at low shear rates and is related to the mud’s low-shear viscosity and elastic properties. Oil-mud polymers may be added to enhance viscosity and cuttings-carrying (hole cleaning) capacity. Care must be taken when adding these products, as over-treatment results in extremely high viscosities. Often, these products will not fully yield until they reach a certain temperature. It is very difficult to decrease excessive viscosities caused by adding these polymers.
Alternative viscosity builders are a class of fatty acid dimers and trimers, which appear to build low-shear viscosity with less effect at high-shear rates. These are particularly effective in deviated wells to ensure the suspension of weighting materials and cuttings during periods of little or no circulation. Rheology modifiers of this type typically increase low-shear-rate viscosity values as determined by the three and 6-rpm readings on a viscometer. Barite can “sag” (Barite Sag) or slide down the hole, especially on deviated wells; these additives will minimize or eliminate this “sag”. Increases in total mud viscosity are avoided when using these additives.
Weighting Agents
used to increase the density of the oil mud. The most commonly used weighting agent is barite. A mud weight of around 21.0 lb/gal is the highest achievable with barite. Hematite, with an s.g. of 4.85, can also be used to increase the density of the oil mud. A mud weight of around 24.0 lb/gal can be achieved with hematite. For the same mud weight, the solids content of the oil mud weighted with hematite will have a lower solids content than that weighted with barite because of the higher S.G. of the hematite.