Like Cement Slurry Acceleration, the mechanisms of Portland cement retardation are still a subject of controversy. Several theories have been proposed, but none fully explains the retardation process by itself. Two principal factors must be considered: the chemical nature of the cement retarders and the cement phase (silicate or aluminate) upon which the retarder acts. The four principal theories are summarized below.
Cement Retarders Mechanisms
- Adsorption theory: The retarder adsorbs onto the surfaces of the hydration products, thereby inhibiting contact with water.
- Precipitation theory: The retarder reacts with calcium ions, hydroxyl ions, or both in the aqueous phase, forming an insoluble and impermeable layer around the cement grains.
- Nucleation theory: The retarder adsorbs onto the nuclei of hydration products, arresting their future growth.
- Complexation theory: The retarder chelates the calcium ions, preventing the formation of nuclei.
Lignosulfonate Cement Retarders
The most commonly used retarders for well cements are the sodium and calcium salts of lignosulfonic acids (Fig. 1). Lignosulfonates are polymers derived from wood pulp; therefore, they are usually unrefined and contain various amounts of saccharide compounds. The average molecular weight varies from about 20,000 to 30,000. Purified lignosulfonates have much less retarding power; therefore, the retarding action is often attributed to the impurities in the bulk material. Such impurities include low-molecular-weight carbohydrates such as pentoses (xylose and arabinose), hexoses (mannose, glucose, fructose, rhamnose, and galactose), and aldonic acids (especially xylonic and gluconic acids) (Chatterji, 1967; Milestone, 1976; 1979).
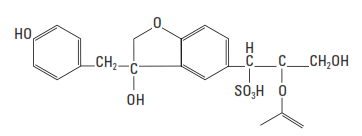
Lignosulfonate retarders are effective with all Portland cement and are generally added in concentrations ranging from 0.1% to 1.5% BWOC (Fig. 2). Depending upon the lignosulfate retarders’ carbohydrate content and chemical structure (e.g., molecular weight distribution and degree of sulfonation) and the nature of the cement, they are effective to about 250°F [122°C] bottom hole circulating temperature (BHCT). When blended with sodium borate, the effective temperature range of lignosulfonates can be extended to as high as 600°F [315°C] BHCT.
![effect of a lignosulfonate retarder on a 15.8-lbm/ gal [1,900 kg/m3] Class G cement](https://www.drillingmanual.com/wp-content/uploads/2021/04/image-38.png)
gal [1,900 kg/m3] Class G cement
Lignosulfonate retarders predominantly affect the kinetics of C3S hydration; however, their effects upon C3A hydration are not insignificant (Stein, 1961b; Angstadt and Hurley, 1963). The retardation mechanism of the lignosulfonates is generally thought to be a combination of the adsorption and nucleation theories.
Lignosulfonate retarders perform best with low-C3A cements. When C3A is hydrated in the presence of organic additives such as lignosulfonates, the solution concentration of the additives quickly falls. C3A hydration products have a much stronger adsorptive effect than those of C3S (Blank et al., 1963; Rossington and Runk, 1968). In a Portland cement system, C3A hydration can prevent a significant amount of lignosulfonate from reaching the surfaces of C3S hydration products; as a result, the cement retarders are less efficient (Young, 1969).
Ramachandran (1972) showed that the sulfonate and hydroxyl groups adsorb onto the C-S-H phase layer. As a result, the permeability of the C-S-H phase is reduced (Ciach and Swenson, 1971). A waterproofing mechanism, preventing further significant hydration, has also been proposed (Jennings et al., 1986).
Some of the lignosulfonate cement retarders remain in the aqueous phase. It may be in a free state or linked to calcium ions through electrostatic interactions. At low lignosulfonate concentrations, the crystal growth (and probably the nucleation) of calcium hydroxide is inhibited (Jawed et al., 1979). Although the same experiment has not yet been performed with C-S-H phase, a similar result would be expected. A significant change in the size and morphology of the calcium hydroxide crystals is observed when C3S is hydrated in the presence of lignosulfonates (Berger and McGregor, 1972). These results suggest that, if the nucleation and crystal growth of the hydration products are hindered by the lignosulfonate, the hydration rate of C3S will be similarly affected.
Hydroxycarboxylic acids
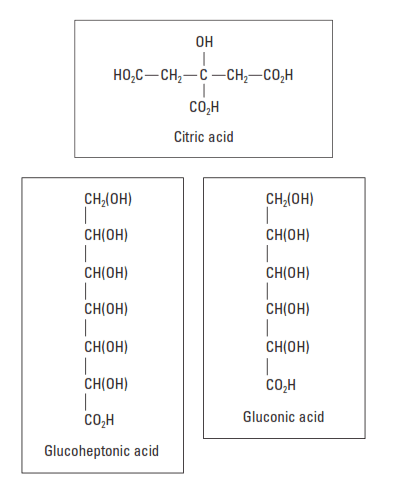
Hydroxycarboxylic acids contain hydroxyl and carboxyl groups in their molecular structures (Fig. 3). Gluconate and glucoheptonate salts and tartaric acid are the most widely used materials in this category. They have a powerful retarding action and can easily cause overretardation at BHCTs less than 200°F [93°C]. As shown in Fig. 4, these materials are efficient to temperatures approaching 300°F [150°C].
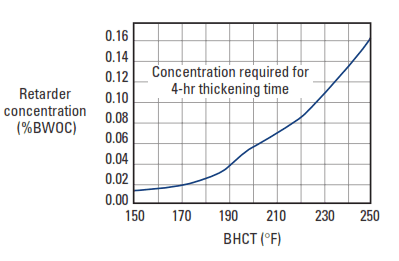
with a 15.6-lbm/gal [1,870-kg/m3] Class A cement slurry.
Another hydroxycarboxylic acid with a strong retarding effect is citric acid. Citric acid is also an effective cement dispersant and is normally used at concentrations between 0.1% and 0.3% BWOC.
The retarding action of hydroxycarboxylic acids cement retarders and their salts is generally attributed to the presence of alpha- or beta-hydroxycarboxylic groups (HO-C-CO2H and HO-C-C-CO2H, respectively) that are capable of strongly chelating a metal cation such as calcium (Double, 1983). Highly stable five- or six-membered rings form, partially adsorb onto the hydrated cement surface, and poison the nucleation sites of hydration products. Like the lignosulfonates, hydroxycarboxylic acids are more efficient with low-C3A cement.
Saccharide compounds
Saccharide compounds (also called sugars) are excellent Portland cement retarders (Fig. 3-8). The best performers contain a five-membered ring (e.g., sucrose and raffinose) (Bruere, 1966; Previte, 1971; Thomas and Birchall, 1983). Such compounds are not commonly used in well cementing, because the degree of retardation is very sensitive to small variations in concentration.
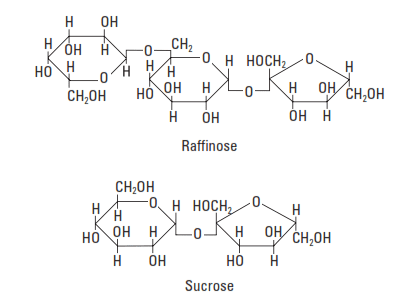
The retarding action of saccharide compounds depends upon their susceptibility to degradation by alkaline hydrolysis. The sugars are converted to saccharinic acids containing alpha-hydroxycarbonyl groups (HO-C-C=O), which adsorb strongly onto C-S-H phase surfaces (Taplin, 1960). Hydration is inhibited when the C-S-H phase nucleation sites are inactivated (poisoned) by the adsorbed sugar acid anions (Milestone, 1979)
Cellulose Derivatives Cement Retarders
Cellulose polymers are polysaccharides derived from wood or other plants. They are stable in the alkaline environment of cement slurries. Set retardation occurs when the polymer adsorbs onto the hydrated cement surfaces. The active sites for adsorption are the ethylene-oxide links and carboxyl groups.
The most common cellulosic retarder is carboxymethylhydroxyethylcellulose (CMHEC) (Shell and Wynn, 1958). Its molecular structure is shown in Fig. 6. CMHEC is an effective retarder at temperatures up to about 250°F [121°C] (Rust and Wood, 1966). Typical performance data are presented in Fig. 7.
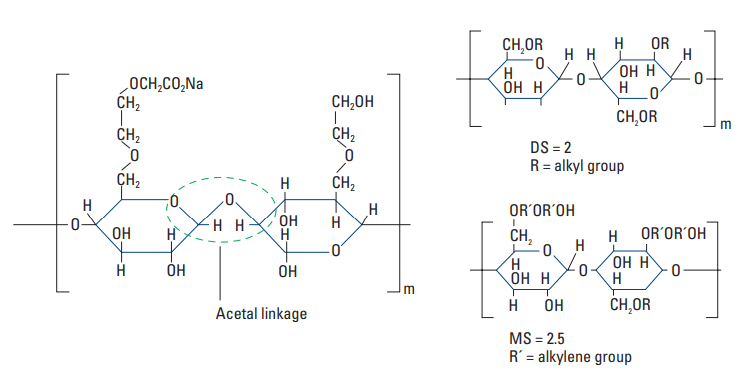
substitution (MS) concepts
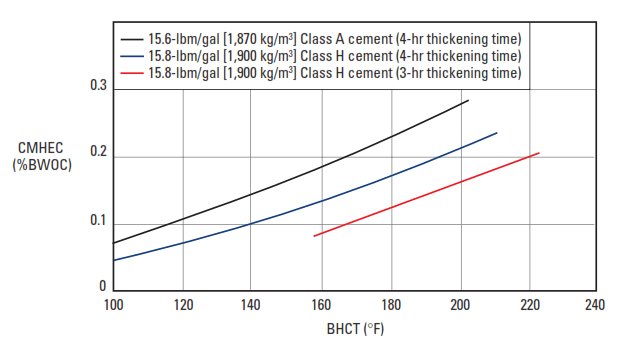
A number of secondary effects are observed with CMHEC. It is often used as a fluid-loss control agent. In addition, CMHEC significantly increases the viscosity of the slurry.
Organophosphonates
In the 1980s, alkylene phosphonic acids and their salts were identified as set-retarding additives for well cements (Nelson, 1984; Sutton et al., 1985; Childs et al., 1986; Nelson, 1987). Phosphomethylated compounds containing quaternary ammonium groups (Crump and Wilson, 1984), as well as N-phosphonomethyl iminodiacetic acid (Huddleston, 1995) are also efficient. Such materials have excellent hydrolytic stability and, depending upon the molecular backbone, are effective to circulating temperatures as high as 450°F [232°C].
Organophosphonates cement retarders are advantageous for well cementing because of their apparent insensitivity to subtle variations in cement composition and their tendency to lower the viscosity of high-density cement slurries. The mechanism of action involves the adsorption of phosphonate groups (Fig. 8) onto the nuclei of cement hydrates, thus hindering their growth.
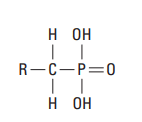
Methylenephosphonic acid derivatives can be used to prepare cement slurries with very long thickening times. The set can then be activated when needed (e.g., by an aqueous solution of sodium silicate) (Childs et al., 1987). They can also be used to retard ultrafine cement (Blaine fineness of greater than 6,000 cm2 /g) at circulating temperatures up to about 400°F [204°C] (Brothers, 1994; Rodrigues and Lindsey, 1995). Ultrafine cement slurries are used for squeeze cementing and well repair.
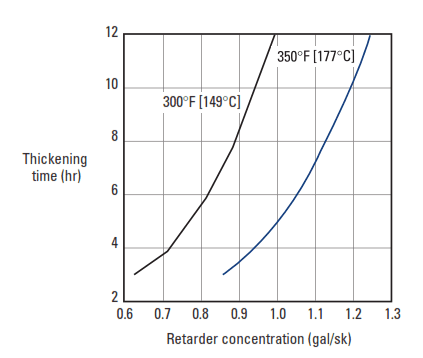
At temperatures above about 230°F [110°C], methylene phosphonic acid derivatives are poor retarders of the aluminate (C3A) and aluminoferrite (C4AF) phases, but they strongly retard the silicate phases (C3S and C2S) (unpublished data, M. Michaux, 1993). As a result, thickening times are short, and compressive strength development is slow. Adding a borate salt to the retarder formulation solves this problem. At an appropriate phosphonate/borate ratio, longer thickening times and swift compressive strength development can be achieved at circulating temperatures up to 450°F [232°C] (Nelson, 1984; Nelson and Casabonne, 1992; Barlet-Gouédard et al., 1996; Casabonne et al., 1996) (Fig. 9).
Inorganic compounds
Many inorganic compounds retard the hydration of Portland cement. The major classes of materials are listed below.
- Acids and salts thereof: boric, phosphoric, hydrofluoric, and chromic
- Sodium chloride: concentrations greater than 20% BWOW
- Oxides: zinc and lead
In well cementing, zinc oxide (ZnO) is sometimes used for retarding thixotropic cements, because it does not affect the cement slurry rheology ( Check also oil well cement properties)or the hydration of the C3A-gypsum system (Ramachandran, 1986). The retardation effect of ZnO is attributed to the precipitation of zinc hydroxide onto the cement grains (Arliguie and Grandet, 1985). Zn(OH)2 has a low solubility (Ks = 1.8.10–14) and is deposited as a colloidal gel; consequently, the precipitate has a low permeability. The retardation effect ends when the gelatinous zinc hydroxide eventually transforms to crystalline calcium hydroxyzincate.
2Zn(OH)2 + 2OH– + Ca2+ + 2H2O→CaZn2(OH)6 • 2H2O (1)
Sodium tetraborate decahydrate (borax: Na2B4O7 • 10H2O) is commonly used as a “retarder aid.” It has the ability to extend the effective temperature range of most lignosulfonate retarders to as high as 600°F [315°C]; however, it can be detrimental to the effectiveness of cellulosic and polyamine fluid-loss additives.
Ref: Schlumberger Well Cementing, Erik B. Nelson and Dominique Guillot