The alkalinity is important in Water-Based Mud because it can affect the performance of some treating chemicals in the water-based fluid, especially if they require alkaline conditions for effective performance. Sodium or Potassium Hydroxide usually provides the hydroxyl ions (OH–) for adjusting the pH of water-based fluids ( see also pH Test Procedures). However, carbonate (CO3 2–) and bicarbonate (HCO3 –) ions also provide fluid alkalinity, along with the formation of contaminants (e.g., H2 S or CO2 gas). Based on the above pf mf drilling fluid / Drilling Mud Alkalinity Test became so important to be done on the drilling rig.

Why are drilling fluid alkalinity tests Pf, Mf important?
However, fluid alkalinity from carbonates and bicarbonates can often harm mud properties, resulting in high viscosities (see also Marsh Funnel Test Procedures), poor fluid loss control, and clay flocculation. The reason for measuring the alkalinity of mud filtrate samples is to determine the amount of carbonate and bicarbonate contamination in the mud system so that effective treatments can be made to restore mud properties, as detailed at the end of the test procedure.
Pf, Mf Drilling Fluid Test
What equipment is required for measuring filtration alkalinity ( Pf, Mf Drilling fluid)?
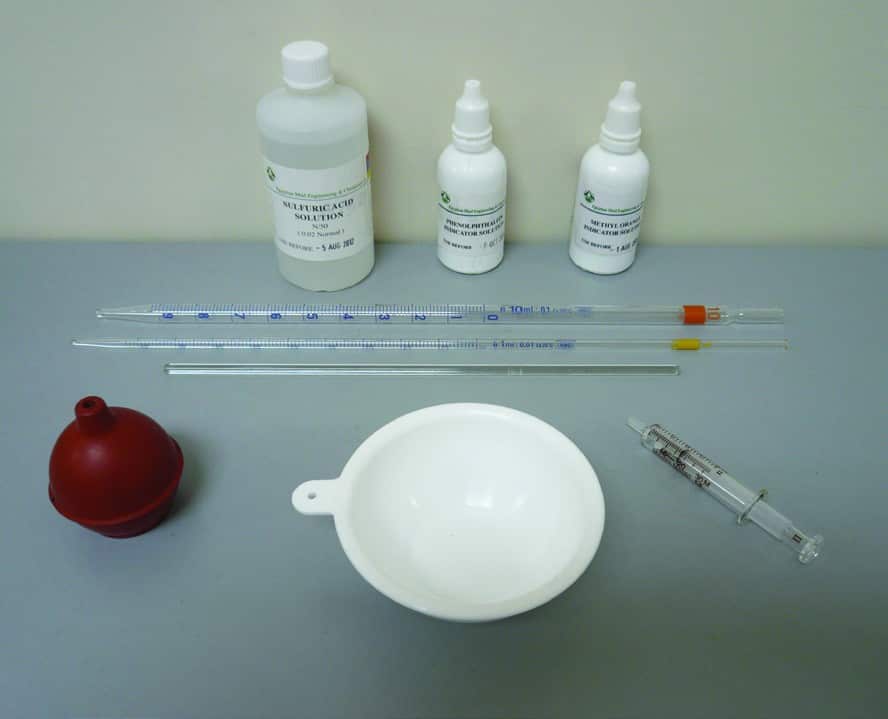
Equipment used for measuring Filtrate Alkalinity
- Sulfuric Acid (0.02N)
- Phenolphthalein indicator solution.
- Methyl Orange indicator solution.
- Titration dish (white ceramic or white polyethylene crucible)
- Graduated Pipette 1 ml
- Graduated Pipette 10 ml
- Glass stirring rod
- Syringe 1 ml
The procedure for measuring Filtrate Alkalinity (Pf, Mf drilling fluid) is as follows:
- Transfer 1 ml mud filtrate into a titration dish using a 1 ml pipette.
- Stir with a glass rod while adding a few drops Phenolphthalein indicator solution (A pink color will appear if alkalinity is present).
- Titrate with 0.02N Sulfuric Acid using a 1 ml graduated pipette while stirring the contents of the titration dish with the glass rod until the pink color disappears, which occurs when pH 8.3 is reached.
- Report the Phenolphthalein alkalinity of the filtrate, Pf, as the volume (ml) of 0.02N Sulfuric Acid (per milliliter of filtrate) required to reach the Phenolphthalein end point (8.3).
- To the same sample that has just been titrated to the Pf endpoint, add a few drops of Methyl Orange indicator solution, titrate with 0.02N Sulfuric Acid using a 1 ml graduated pipette while stirring the contents of the titration dish with the glass rod until the yellow/orange color changes to red, which occurs when pH 4.3 is reached.
- Report the Methyl Orange alkalinity of the filtrate, Mf, as the total volume (ml) of 0.02N Sulfuric Acid (per milliliter of filtrate) required to reach the Methyl Orange endpoint (i.e. including the volume for measuring Pf).
It is important to be aware that anionic thinners and fluid loss additives will contribute to the Mf alkalinity value and may also mask the endpoint color change, so the accuracy of the results may be affected when these additives are present in significant quantities. However, the presence of hydroxide (OH–), carbonate (CO3 2-), and bicarbonate (HCO3 –) ions can be estimated from the alkalinity results for the mud filtrate (Pf and Mf), as shown below.
Method for determining hydroxide, carbonate, and bicarbonate ion concentrations in water-based fluids.
a. If Pf = 0, then alkalinity is due to bicarbonate ions.
[Bicarbonate] = Mf x 1,220 mg/l
b. If Pf = Mf , then alkalinity is due to hydroxide ions.
[Hydroxide] = Pf x 340 mg/l
c. If 2Pf < Mf , then alkalinity is due to carbonate and bicarbonate ions
[Carbonate] = 2Pf x 600 mg/l
[Bicarbonate] = (Mf – 2Pf) x 1,220 mg/l
d. If 2Pf = Mf , then alkalinity is due to carbonate ions only.
[Carbonate] = Mf x 600 mg/l
e. If 2Pf > Mf , then alkalinity is due to hydroxide and carbonate ions.
[Hydroxide] = (2Pf – Mf ) x 340 mg/l
[Carbonate] = (Mf – Pf ) x 1,200 mg/l
The pf, mf drilling fluid filtrate alkalinity measurements will indicate the presence and concentrations of carbonate and/ or bicarbonate contamination, enabling effective treatments to be made if mud properties are being affected (e.g., high viscosity, poor fluid loss control, clay flocculation). The presence of bicarbonate ions often makes the mud system difficult to control. However, this is relatively simple to remedy because bicarbonate ions can be converted to carbonate ions by adding hydroxide ions in the form of Sodium or Calcium Hydroxide.
Drilling Mud/fluid Alkalinity Test Pf, Mf Procedures
What Is The Required Equipment For Measuring Drilling Mud Alkalinity?
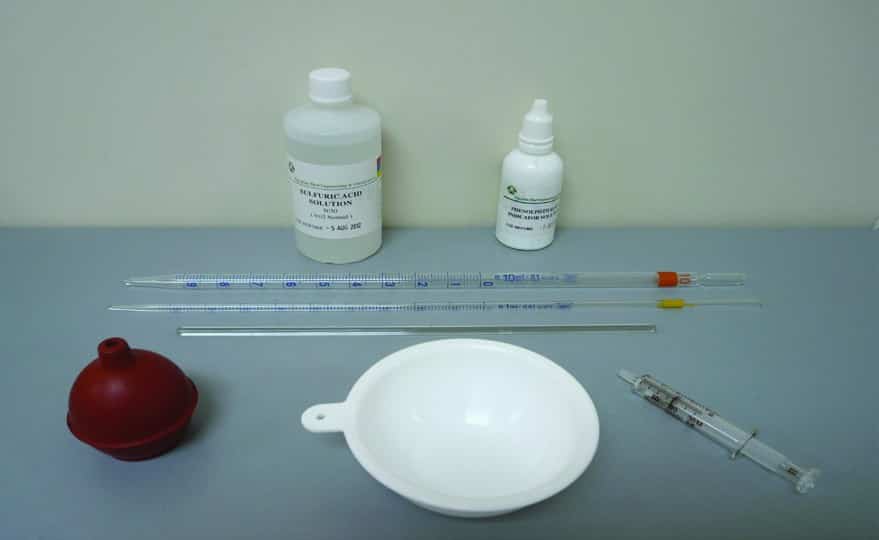
The procedure for measuring Mud Alkalinity (Pm) is similar to that for measuring Filtrate Alkalinity, using the following equipment:
- Sulfuric Acid (0.02N)
- Phenolphthalein indicator solution
- Titration dish (white ceramic or white polyethylene crucible)
- Syringe 1 ml
- Graduated Pipette 1 ml
- Graduated Pipette 10 ml
- Glass Stirring Rod or Magnetic Stirrer with Bar Magnet
The procedure for measuring Mud Alkalinity (Pm) is as follows:
- Add approximately 25 ml distilled water to a clean titration dish and place on a magnetic stirrer, if available.
- Transfer 1 ml mud sample into a titration dish using a 1 ml pipette.
- Use a magnetic stirrer with bar magnet to agitate the fluid in the titrating dish while adding the test chemicals, otherwise use a glass stirring rod.
- Add a few drops Phenolphthalein indicator solution (a pink color will appear if alkalinity is present).
- Titrate with 0.02N Sulfuric Acid using a 1 ml graduated pipette while stirring the contents of the titration dish with the glass rod until the pink color disappears, which occurs when pH 8.3 is reached.
- The volume (mls) of 0.02N Sulfuric Acid (per milliliter of mud) required to restore the original color of the diluted mud sample is the Phenolphthalein alkalinity of the mud, Pm.
If the mud is contaminated with cement, then the titrated sample will gradually turn pink again after a few seconds. If this is the case then the titration should be performed as quickly as possible until the pink color first disappears, in order to eliminate distortion of the Pm reading due to cement contamination.
Alkalinity Pf Mf Testing For Water-Based Drilling Fluid:
- Objective: Determine the alkalinity (Pm) of a water-based mud (WBM).
- Unit: mL
- Example: Pm = 1.2 mL of 0.02N (N/50) sulfuric acid solution
Equipment For Water-Based Drilling Mud Fluid Alkalinity Testing Pf, Mf:
- Titration dish
- 3-mL syringe (without needle)
- 5-mL pipette
- Stirring rod
- 50-mL graduated cylinder (250-mL for lime muds)
- 0.02N (N/50) sulfuric acid solution
- Phenolphthalein indicator solution
- Distilled water
Water-Based Drilling Mud Alkalinity Testing Procedures:
- Collect a fluid sample.
- Transfer 1 mL of the sample to the titration dish using the syringe.
- Add 50 mL of distilled water to the titration dish and stir. Note the color of the mixture for Step
Note: For lime muds, use 200 mL of distilled water. - Add 10 to 15 drops of phenolphthalein indicator solution to the titration dish and stir.
- If A pink or red color develops, Go to Step 5
- If There is no color change, Pm equals zero. Go to Step 6.
- Add the sulfuric acid solution one drop at a time to the titration dish until the color changes from pink or red to the original color. 6. Record the amount of sulfuric acid solution used (in mL) as Pm.
Alkalinity Testing Pf Mf For Oil-Based Drilling Mud / Synthetics Drilling Fluids
- Objective: Determine the whole-mud alkalinity and lime content of oil-based mud (OBM) or synthetics.
- Unit: mL
- Example: Alkalinity = 1.8 mL of 0.1N (N/10) sulfuric acid solution
Equipment For Oil-Based Drilling Fluid Alkalinity Testing Pf Mf
- 500-mL Erlenmeyer flask with a rubber stopper or a pint jar with a lid
- 3-mL disposable syringe
- 50-mL graduated cylinder
- 250-mL graduated cylinder
- Two 1-mL pipettes
- Two 5-mL pipettes
- Arcosol PNP® solvent
Note: The base fluid can be used if the solvent is unavailable. - Distilled water
Note: If distilled water is unavailable, nondistilled water can be used. The pH of the water must be approximately 7. - Phenolphthalein indicator solution C 0.1N (N/10) sulfuric acid solution C 0.1N (N/10) sodium hydroxide
Oil-Based Drilling Mud Alkalinity Testing Pf Mf Procedure
- Collect a drilling fluid sample.
- Measure 100 mL of Arcosol PNP solvent into the Erlenmeyer flask.
- Add 1.0 mL of the fluid sample to the Erlenmeyer flask using the syringe.
- Stopper the flask and shake vigorously.
- Add 200 mL of distilled water and 10 to 15 drops of phenolphthalein indicator solution to the flask.
- Stopper the flask and shake vigorously for a minimum of two minutes.
- Allow the phases to separate.
- If A pink or red color develops, Go to Step 8
- If there is no color change, Alkalinity equals zero. Go to Step 16.
- Add 3 mL of sulfuric acid solution to the flask using the 5-mL pipette.
- Stopper the flask and shake vigorously.
- Allow the phases to separate.
- If the solution remains pink, Go to Step 11
- If the solution turns colorless, Go to Step 12.
- Continue to add sulfuric acid solution in 3-mL increments until the pink color disappears. Note: Shake the solution after each addition of sulfuric acid.
- Record the volume of sulfuric acid used in mL.
- Back titrate with sodium hydroxide using the 1-mL pipette until the pink color first reappears and remains.
Note: Shake the solution after each addition of sodium hydroxide. Add sodium hydroxide only until the pink color reappears. - Record the volume of sodium hydroxide used in mL.
- Calculate alkalinity.
Alkalinity = mL N/10 sulfuric acid – mL N/10 sodium hydroxide - Calculate excess lime pounds per barrel of mud.
Excess lime, lb/bbl = 1.3 × alkalinity
Alkalinity Test: Filtrate (Pf /Mf )
- Objective: Determine the amounts of soluble ions that contribute to alkalinity in a water-based drilling fluid. Note: If the mud contains high concentrations of organic thinners (i.e., CARBONOX), use the alternate filtrate alkalinity (P1/P2) method.
- Unit: mL
- Example: Pf = 0.3 mL of 0.02N (N/50) sulfuric acid solution Mf = 1.3 mL of 0.02N (N/50) sulfuric acid solution
Alkalinity Test Equipment
- Titration dish
- 1-mL pipette C 2-mL pipette C 5-mL pipette C Stirring rod
- Distilled water
- 0.02N (N/50) sulfuric acid solution
- Phenolphthalein indicator solution
- Methyl orange indicator solution
Note: As an option, use methyl purple indicator solution or bromocresol green.
Procedure For Alkalinity Test
- Collect a filtrate sample using the API filtrate (LPLT) method.
- Transfer 1 mL of the filtrate to the titration dish using the 1 mL pipette.
- Add 10 to 15 drops of phenolphthalein indicator solution to the titration dish.
- If there is a color change, Go to Step 4.
- If there is no color change, Pf is zero. Go to Step 6.
- Add the sulfuric acid solution slowly to the titration dish (using the 2- or 5-mL pipette) until the color changes from pink or red to the original filtrate color.
- Record the amount of sulfuric acid solution used in mL as Pf.
- Add 10 to 15 drops of methyl orange indicator solution to the filtrate mixture.
- Continue titrating with the sulfuric acid solution until the color changes from orange to salmon pink.
- Record the total amount of sulfuric acid solution used, including the amount from the Pf test, as the Mf value. –
- Calculate the concentration of hydroxyl (OH-), carbonate (CO3 -2 ), and bicarbonate (HCO3- ) ions using the following table.
| Concentration, mg/L | |||
| Criteria | OH– | CO -2 | HCO3 – |
| Pf = 0 | 0 | 0 | 1,220 Mf |
| 2Pf < Mf | 0 | 1,200 Pf | 1,220 (Mf – 2Pf) |
| 2Pf = Mf | 0 | 1,200 Pf | 0 |
| 2Pf > Mf | 340 (2Pf – Mf) | 1,200 (Mf – Pf ) | 0 |
| Pf = Mf | 340 Mf | 0 | 0 |
Excess lime, lb/bbl = 0.26 x [Pm & ( Pf x Fw )]
Excess lime, kg/m 3 ‘ 0.74 x [Pm & ( Pf x Fw )]
An approximation of excess lime can be obtained by:
Excess lime, lb/bbl = (Pm – Pf )/4
Excess lime, kg/m 3 = (Pm – Pf ) x 0.7
Where:
- Pf is the phenolphthalein endpoint of the filtrate
- Pm is the phenolphthalein endpoint of the mud
- Fw is the water fraction
Alkalinity: Alternate (P1 /P2)
- Objective: Determine the amounts of soluble ions that contribute to alkalinity in a water-based drilling fluid.
- Unit: mL
- Example: P1 = 11.5 mL of 0.02N (N/50) hydrochloric acid solution
P2 = 9.8 mL of 0.02N (N/50) hydrochloric acid solution
Testing Equipment
- 1-mL volumetric pipette
- 2-mL volumetric pipette
- Titration dish
- 25-mL graduated cylinder
- 5-mL or 10-mL graduated cylinder
- Stirring rod
- 3-mL syringe
- Distilled water
- Barium chloride solution (10 percent, neutralized to pH 7 with NaOH)
- Phenolphthalein indicator solution
- 0.02N (N/50) hydrochloric acid solution
- 0.1N (N/10) sodium hydroxide solution
Procedure For Testing
- Collect a filtrate sample using the API filtrate (LPLT) method.
- Determine the Pf alkalinity of the sample using Steps 2 through 5 of the Pf /Mf procedure. Note: Substitute hydrochloric acid for sulfuric acid solution.
- Determine the P1 alkalinity.
- Transfer 1.0 mL of filtrate to the titration dish.
- Add 24 mL of distilled water to the titration dish
- Add exactly 2.0 mL of sodium hydroxide solution to the titration dish using the volumetric pipette
- Add 3 mL of barium chloride solution to the titration dish using the 3-mL syringe. Warning: Barium chloride is extremely poisonous. Be sure to use a syringe, and not a pipette, to add the barium chloride solution to the titration dish
- Add 2 to 4 drops of phenolphthalein indicator solution while stirring the contents of the titration dish
- Titrate the mixture with the hydrochloric acid solution (using the 10-mL pipette) until the solution is colorless. Note: If the pink color reappears, do not continue the titration.
- Record the volume of hydrochloric acid solution needed to reach the endpoint as P1
- Determine the P2 alkalinity.
- Add 25 mL of distilled water to a clean titration dish.
- Repeat Steps 3c through 3f to determine P2.
- Record the volume of hydrochloric acid solution needed to reach the endpoint as P2.
- Calculate the concentration of hydroxyl (OH-), carbonate (CO3 -2), or bicarbonate (HCO3- -) ions.
| Concentration, mg/L | |||
| Criteria | OH– | CO -2 | HCO3 – |
| P1 > P2 | 340 (P1 – P2) | 1,200 [Pf – (P1 – P2)] | 0 |
| P1 = P2 | 0 | 1,200 Pf | 0 |
| P1 < P2 | 0 | 1,200 Pf | 1,220 (P2 – P1) |
Lime Content Calculations
The Lime Content can be calculated from the following mud properties, as measured in previous procedures:
- Mud and Filtrate Alkalinities (Pm and Pf)
- Water Fraction from Retort Results
The water fraction is simply the water percentage divided by 100 (i.e., 78% water from the retort results is equivalent to 0.78 Water Fraction).
Estimated Lime (ppb) = 0.26 x [Pm – (Pf x Water Fraction)]