
The mud system used most frequently throughout the industry is the water-based system. Water is the continuous phase, but it may contain oil (i.e., emulsion muds) (also check: Oil Based Mud) or air (i.e., aerated mud) as the discontinuous phase. The oil must remain as segregated droplets and not combine in a distinct ” discontinuous ” phase (Fig. 1).
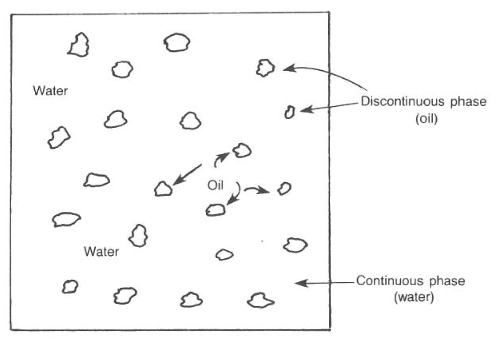
Clear, freshwater is one of the oldest drilling muds used in the industry. We generally use it with no special additives except perhaps a corrosion inhibitor. The freshwater may hydrate formation clays and convert them into “native” mud. We believe that the term “mud” was used when fresh water and surface soils were mixed to develop a viscous fluid that could increase the hole-cleaning characteristics of the fluid.
Composition of WBM
The main phases of WBM are:
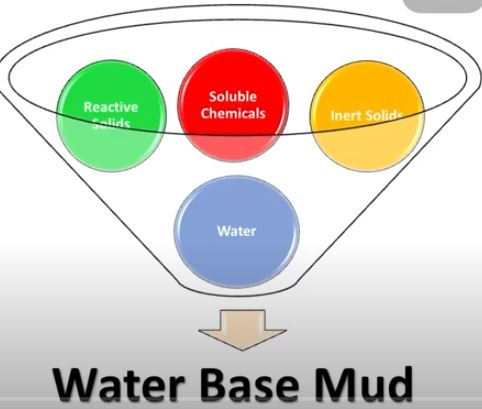
- Water is the drilling fluid’s continuous (liquid) phase (mud). It can be freshwater, brackish water, seawater, saturated salt water, or another type of brine fluid
- Reactive commercial clay solids
- Reactive formation drilled solids
- Inert formation drilled solids
- Inert commercial solids
- Soluble Chemicals

Solids Systems (Solids Tolerance).
We may use the solids concentration in the water-based drilling mud to describe the system. Clearwater mud has virtually no detectable solids. A low-solids system has some solids, although there are a lot of efforts to minimize solids concentration. We usually use a high-solids system while penetrating”mud-making” formations. We shall control the solids content of a high-weight mud within acceptable ranges.
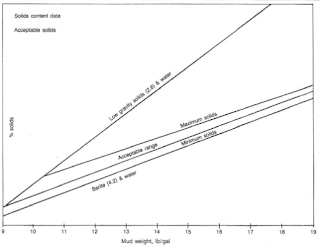
The degree to which formation solids contaminate a water-based drilling mud depends on the characteristics of the continuous phase and the type and amount of clay in the rock cuttings. We may control excess solids by surface mechanical equipment and/or water dilution. Water dilution is the most expensive process for correcting drilled solids accumulation. Fig.5 shows acceptable ranges for mud solids developed by several mud companies.
Water-Based Drilling Mud Types
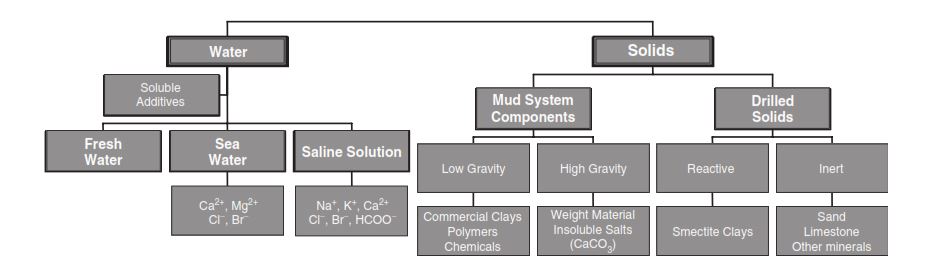
We usually convert basic WBM systems to more complex ones as we deepen the well. The major WBM Types are:
- Inhibited Mud
- Dispersed Mud
- Undispersed Mud
- Flocculated Mud
- Brines
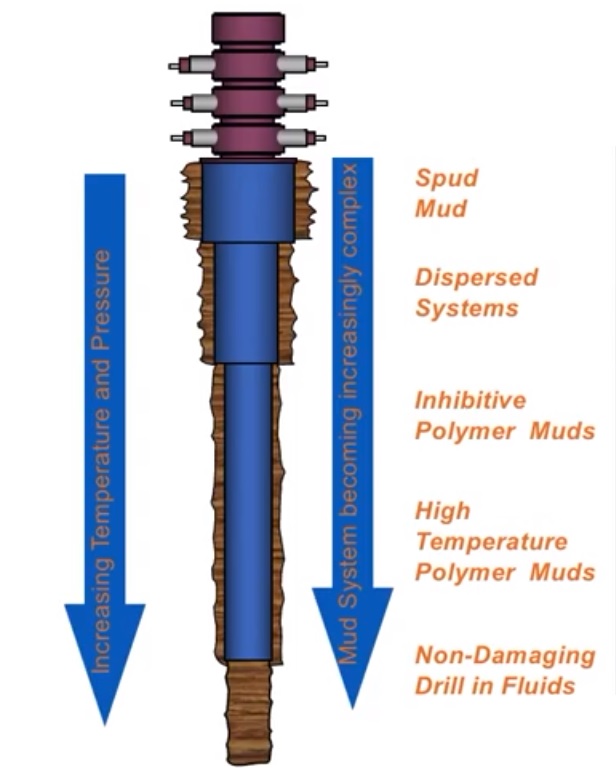
An Inhibited Water-Based Drilling Mud
We often use inhibited water-based mud to minimize hole-sloughing problems. “Inhibition” refers to retarding the rate at which formation clays hydrate. Hydration reduces the structural stability of the borehole, allowing it to fall or slough into the wellbore. Commonly inhibited muds, such as lime muds, use calcium to retard hydration, while others use high concentrations of various salts.
Four common mud systems that can be classified as inhibited muds are
- Gyp muds.
- Lime muds.
- Seawater muds.
- Saturated saltwater muds.
Lime Mud
There was wide use of lime mud for many years as inhibited fluids. The hydrated lime, calcium hydroxide, reduces the water attached to the clay structure. High lime-content water-based drilling mud should contain a Pf of 5-9 and a Pm of 2~O to maintain excess lime. We can estimate the amount of undissolved lime with Eq. 1:
Lime = (Pm – Pf )/ 4 Eq.1
Where
- The measurement of the excess lime is in Ib/bbl
- Pm is the phenolphthalein end point of mud
- Pf is the phenolphthalein alkalinity endpoint of filtrate.
In recent years, there has been a wide successful application of low-lime mud in high-temperature wells. High lime concentrations tend to cause clay flocculation in the upper-temperature ranges. Low-lime water-based drilling mud should contain a Pf of 2-5 and a Pm of 12-18. Closely monitoring the mud system is essential to maintain undissolved lime.
Gyp Mud
Generally, the main application of Gypsum (calcium sulfate) water-based drilling mud in gyp and anhydrite formations. Gyp muds are similar to lime muds since they derive their inhibition properties from soluble calcium and require a chemical thinner for viscosity control. These muds function at lower alkalinity ranges than lime muds and contain more soluble calcium.
Seawater muds
This type of water-based drilling mud commonly used in offshore drilling is more economical than freshwater systems in some cases. The convenient source of salt water and the elimination of extra storage space on offshore rigs have made this type of mud very popular. Since seawater contains salt, NaCl inhibits clays’ hydration and dispersion. Alkalinities are extremely important. In addition, closely monitoring them is necessary for the dispersants and clays to react properly.
Saturated salt muds
Saturated salt mud contains a large amount of dissolved salt and can be classified as inhibited mud. However, the major application of saturated salt muds is to drill salt domes or thick stringers of salt. Normally, we accomplish salt contamination and elimination of the development of large cavities in the salt formation by saturating water-based mud with salt.
Dispersed Drilling Mud
A dispersed water-based drilling mud usually uses drilling chemicals that cause the clay platelets to “disperse,” or separate, within the liquid phase (Fig.2). These dispersed muds tend to have better viscosity control and higher viscosity solids tolerance and better filtration control than nondispersed systems. Usually, we use dispersed systems in drilling young, high-activity clays such as those found in the Gulf of Mexico and Nigeria. A common dispersed system is lignosulfonate mud.
Non-Dispersed Water-Based Drilling Mud.
Nondispersed muds are often associated with low solids concentrations and relatively low-density weights. These muds do not contain chemical dispersants and are formulated normally with a minimum amount of bentonite. The systems use a polymer(s) that extends the effects of small amounts of bentonite and selectively flocculates undesirable drilled solids. For low-solids nondispersed mud to function effectively, we should closely monitor solids control equipment, alkalinities, and bentonite concentrations.
Flocculated Mud Systems.
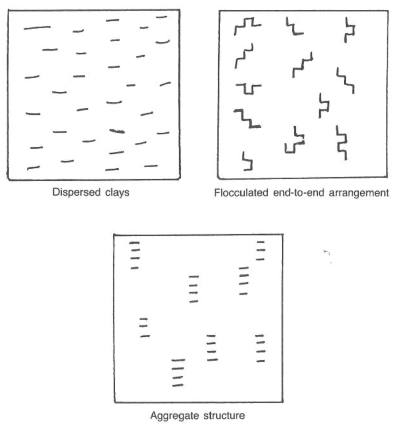
Flocculated water-based drilling mud causes the clay platelets to arrange themselves in an edge-to-face orientation. This arrangement often occurs with the intrusion of some contaminant. Filtration, viscosity, and gel strengths will increase dramatically once mud flocculates. Chemical agents that decrease the sensitivity of the edge-to-face attraction of clay particles are available. These deflocculants restore the clay particles to a dispersed or aggregated state.
Brines.
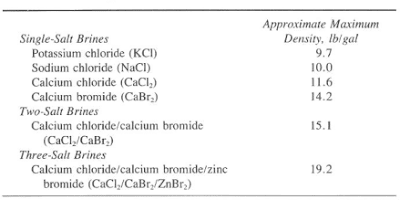
Clearwater and some brine fluids do not require high densities and/or high viscosities. Their main application is completion and workover operations. This is because very low solids and a clean environment are critical. Brines may range from 8.33 to 19.2 Ib/gal. These brines may be single-, two-, or even three-salt compounds such as the following:
- Potassium chloride
- Sodium chloride
- Calcium chloride/calcium bromide
- Calcium chloride/calcium bromide/zinc bromide
Filtration of Brine makeup water is important to eliminate undesirable solids contamination. We usually measure Brine densities at 60°F. Also, if possible, the density calculations shall be done with specific gravity as measured with a hydrometer. We included brine formulation tables and charts in this Appendix.
Generally, we make single-salt brines from freshwater with a single salt to achieve the desired density. In addition, we can control the density by adding salt or freshwater. Table 1 shows some of the more commonly used salt systems.
Potassium chloride brines
We can use them with densities of 9.7 Ib/gal. Potassium chloride brines are excellent workover fluids for water-sensitive formation. The potassium ion is particularly effective at plugging the clay lattice to minimize the hydration of formation clays, as shown in Fig. 3&4.
A sodium chloride system (NaCI)
Due to its availability, the NaCl system is probably the most common brine water in drilling, completion, and workover operations. The maximum density for a sodium chloride brine is 10.0 Ib/gal at 60 °F. Note that a 9.7 Ib/gal density is relatively easy to achieve. The range from 9.7 to 10.0 Ib/gal requires special attention.
Calcium chloride
We commonly use the Calcium Chloride mixtures when there is a requirement for densities from 9.7 to 11.6 Ib/gal. The wide application of the CaCl2 fluid is in workover and completion operations. The freezing (crystallization) point of an 11.6-lb/gal CaCl2 brine is 44°F, which may cause operating difficulties at low surface temperatures.
Brine systems requiring densities of 15.1 Ib/gal use a two-salt mixture of calcium chloride and bromide (CaCI2 and CaBr2). The basic ingredient of this brine is a calcium bromide solution with a density of 14.1 to 14.3 Ib/gal. The density can increase to 15.1 Ib/gal by adding calcium chloride flakes or pellets. We should take care of cold weather formulation. That is because we shall maintain the solution above its freezing point by mixing proper amounts of calcium chloride & bromide.
High-pressure drilling, workovers, and completion operations occasionally require 19.2 Ib/gal densities. Adding calcium chloride and calcium bromide to a zinc bromide solution can achieve a density of 19.2Ib/gal. In addition, we can utilize various concentrations of the three salts to formulate summer and winter blends.
Water-Based Drilling Fluid Additives

Freshwater is often the base fluid when adding many drilling chemicals such as clays, polymers, weight materials, and additives to control various properties.
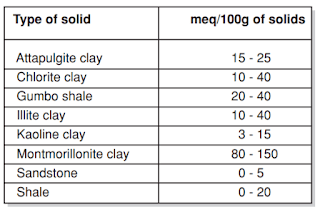
- The clays include sodium and calcium montmorillonite, attapulgite, and subgroups of montmorillonite.
- Polymers cover a broad range of products, such as CMC (carboxymethylcellulose) and HEC (hydroxyethylcellulose).
- Weight materials include barite, galena, iron oxides, and hematite.
- We may utilize special additives for controlling mud properties such as fluid loss, viscosity, gel strength, and pH. Inhibited Water-Based Fluids.
Viscosity Control Additives For Water-Based Mud
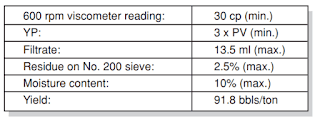
The major application of commercial clay additives is to control the viscosity of water-based drilling fluid. These are graded according to their yield. We can define the clay yield as the number of barrels of 15-centipoise viscosity mud obtained from 1 ton of dry clay. (A 15-centipoise viscosity will support barite). Wyoming bentonite yields about 100 bbl/ton, whereas native clays may only yield 10 bbl/ton. The result would be that the native clay would cause a higher solids content and mud density than the Wyoming bentonite to build the same viscosity. API has laid down the bentonite specifications (Table 4). The salt concentration in the mixed water will affect the clay yield. The hydration and dispersion of the clay can greatly decrease by the presence of Ca2+ and Mg 2+ ions.
How To Control Viscosity
To overcome this problem, we can take various measures:
- Chemical treatment to reduce salt concentration by precipitation.
- Dilution with fresh water.
- Attapulgiton control.
- first hydrate the clay in freshwater, then add the slurry to the salt water.
- Use organic polymer clay. Attapulgite has a different structure from montmorillonite and does not depend on water makeup to build viscosity. It is, however, more expensive and provides poor filter timers (cellulose) to build viscosity

To reduce the viscosity of the mud:
- Lower the solids content.
- Reduce the number of particles per unit volume.
- Neutralize attractive forces between the particles.
Shale shaker screens, desilters, and other mechanical devices will reduce viscosity, but we can also use chemical additives. These chemicals produce negatively charged anions in solution, reducing the positive charge on the edge of the clay plates. This reduces the edge-to-face association and viscosity (thinners or dispersants).
Density Control Additives For Water-Based Mud
Barite (barium sulfate, BaSO4) additives are the primary weighting material used in the water-based drilling fluid. We can easily achieve densities of 9 ppg to 19 ppg by mixing water, clay, and barite. Table 5 shows the API specification for barite. Other weighting materials are calcium carbonate and galena (lead sulfide). The drill solids from the formation will increase the mud density if not separated. This will be discussed under solids control.
Filtration Control Additives
Loss of fluid from the mud occurs when the mud comes into contact with a permeable zone. If the pores are large enough, the first effect will be a spurt loss, followed by the buildup of solids to form a mud cake. The rate at which fluid is lost is a function of the differential pressure, the filter cake thickness, and the filtrate viscosity. Excessive filtration rates and thick wall cake can lead to problems such as:
- Tight spots in the hole
- Differential pipe sticking
- Formation damage due to filtrate invasion
Since a filter cake attains its greatest thickness under static conditions, the mud is tested under static conditions. Due to erosion effects, dynamic filtration results in a thinner mud cake, but the filtration rate will be higher. The aim is to deposit a thin and impermeable filter cake.
How To Control Filtration
Several types of material may be added to the mud to control fluid loss.
- Clays – Bentonite is an effective fluid loss control agent because of its particle size and shape and because it hydrates and compresses under pressure. The particle size distribution is such that most particles will be less than 1 micron. Care should be taken not to remove these small particles by using a centrifuge for solids control.
- Starch – These organic chemicals will swell rapidly and seal off the permeable zones.
- CMC – This is an organic colloid (sodium carboxyl-methyl cellulose). The long-chain molecules can be polymerized into three grades (high, medium, and low viscosity). CMC is thought to control filtration by wedging long-chain polymers into the formation and plugging the pores. CMC works well in most water-based muds but is less effective in high salt concentrations (>50,000 ppm).
- Polyacrylates (Cypan) are long-chain polymers that become absorbed onto the edge of clay particles.
- Lignosuphonates – Similar in action to starch in reducing fluid loss.
- Polyanionic cellulose (Drispac) – An organic compound used to control fluid loss in high salt concentrations and is often used in low solids mud. It may also be used as a viscosifier.
The water loss allowable in any particular area will largely depend on experience. As the well is drilled, the fluid loss must be adjusted as new formations are penetrated. The surface hole may be drilled with a fluid loss of 20 cc, but across productive formations, it will be reduced (down to possibly five ccs). Control over fluid loss depends on correctly adding chemicals and dispersing the clay solids. Fluid loss control agents may act as thinners or viscosifiers under certain circumstances and react unfavorably with other chemicals in the mud.
PH Control Additives For Water-Based Drilling Fluid

Caustic soda NaOH is the major additive used to keep the pH of the mud high. This is desirable to prevent corrosion and hydrogen embrittlement. The pH of most mud lies between 9.5 and 10.5. Caustic potash, KOH, and slaked lime Ca(OH)2 may also be used.
Water Based Mud Contaminants
Various substances may enter the mud, hurting its quality and reducing efficiency. These contaminants must be removed. The main contaminants are listed below:
- Calcium(Ca2+)-may enter from cement, gypsum, lime, or salt water. It reduces the viscosity-building properties of bentonite. It is usually removed from freshwater muds by adding soda ash Na2CO3, which forms insoluble CaCO3. The pH is normally too high if calcium is present in the mud.
- Carbon dioxide (CO2) – is present in formations that, when entrained in the mud, can cause adverse filtration and gelation characteristics. To remove CO2, calcium hydroxide can be added to precipitate CaCO3.
- Hydrogen sulfide (H2S) – is present in formations. Highly toxic gas also causes hydrogen embrittlement of steel pipes. Add NaOH to keep the pH high and form sodium sulfide. If the pH is allowed to drop, the sulfide reverts to H2S.
- Oxygen (O2) – entrained into the mud in surface pits, causes corrosion and pitting of steel pipe. Sodium sulfite (Na2SO3) is added at the surface to remove the Oxygen.
Water-Based Mud Testing
There are six main tests for the WBM, which are as follows:
- Mud Density Test.
- Rheology Test.
- API Filter Press Test.
- HPHT Filter Press Test.
- Sand Content Test.
- Retort Analysis.
We previously discussed the procedure for WBM tests in a previous article according to API RP 13B-1.