Shale has become a significant challenge for the oil and gas industry due to its sensitivity to drilling activities. It is susceptible to wellbore instability, bit balling, and dispersion of shale particles in drilling fluids. Over the years, many oil and gas companies developed various types of shale inhibitors to overcome the shale problems. The most commonly used method to inhibit shale is through water-based drilling fluid. This technique involves using mud or aqueous solutions to create a barrier between the shale and the drill bit. This will prevent the reactive clays within the shale from interacting with the drill bit. The drilling mud is formulated to reduce the hydrogen bonding between clay minerals while providing excellent lubrication at depth. Additionally, it limits the penetration of reactive shales into the drill string, thereby helping to maintain a stable wellbore.
High-performance shale inhibitors have been developed to maintain stable wellbores and control shale hydration during drilling operations. These inhibitors use organic compounds to react with clay surfaces and create a layer of inhibition. Furthermore, this will prevent further swelling and destabilization of shales. They also help prevent the dispersion of shale particles in oil-based drilling fluids. Furthermore, it will lead to improved performance in extreme conditions like high temperatures and pressures. Organic compounds are a key component of these inhibitors, which offer better performance than water-based drilling fluids.
Petroleum engineers have found that high-performance shale inhibitors can improve rolling recovery rates, making subterranean drilling operations more efficient. Studies have been published in journals like J. Energy Resour., Energy Fuels, and Society of Petroleum Engineers Hot-Rolling Recovery Tests (SPE HTRT).
Why We Need the Inhibition?
The cost to the industry of hole problems and lost time incidents caused by shale hydration is immense. There was a development in many products and systems through extensive research to improve wellbore stability and prevent hydration of cuttings. Since there are different shale types and various ways shale hydration and instability can occur, several techniques are available to mitigate the problems that arise. These techniques will be explained in detail below.
Shale Inhibitor Mechanisms
The table below tabulates the range of the inhibition mechanisms and typical product types:
| Inhibitor Process | Shale Inhibitor Mechanism | Typical shale inhibitor Products |
| Cation Exchange | Cations like non-hydrated K+ replace hydrated cations on C.E. sites. | KCl (Some organic cations are also used) |
| Encapsulation | High molecular weight adsorptive polymers coat the cuttings and wellbore surfaces. Most effectively used against cuttings dispersion. | Partially Hydrolyzed PolyAcrylamide (PHPA) |
| Reduction of Water Permeation Rate into Shale | Increased fluid viscosity results in decreased rate of flow into shale pores. Only relatively low molecular weight compounds can flow through tiny pores in shale. | Polyglycerols, Polyalkylene Glycols, Methyl-Glucoside, |
| Replacement of Inter-Layer Water | Using strong solutions of salt or polyhydroxy compounds in the mud reduces vapor pressure (Water Activity), slowing or reversing the transfer of water molecules into shale. | Polyalkylene Glycols, Amine-Capped PAGs |
| Reduction or Reversal of Osmotic Transfer of Water into Shale | NaCl, CaCl2, Potassium formate, Methylglucoside | NaCl, CaCl2 , Potassium formate, Methylglucoside |
| Plugging of Shale Pores to Reduce Pore Pressure Penetration | Some wellbores collapse when mud filtrate enters cracks and micro-fractures. Plasticized particles bridge, coalesce, and seal the fractures | Highly cross-linked polymer microgels, Cloud Point Glycols |
| Sealing of Shale Micro-Fractures | The water between clay sheets is replaced by more strongly adsorbing organic molecules (This is often combined with cation exchange). | Asphalt or Gilsonite particles. Sulfonated Asphalt |
| Reactions with Clays in the Shale to Produce Inter-Particle Cement | Micro-colloids enter and plug the tiny pores in the shale. Some polyglycols may come out of the solution after penetrating the shale and warming up. | High Lime muds, Sodium Silicate solutions (Potassium Silicate is sometimes used) |
How Does Cation Exchange Provide Shale Inhibition?
Cation Exchange of hydrated cations by potassium has been described earlier. Potassium Chloride as a shale inhibitor is very effective at inhibiting the hydration of shales with a high cation exchange capacity (i.e., shales containing a substantial concentration of montmorillonite and/or illite). The shale inhibitor used for such a mechanism
Does Encapsulation Make Shale Inhibition?
Encapsulation by the partially hydrolyzed polyacrylamide is effective for dealing with highly dispersive shales, such as micaceous siltstones & kaolin-rich formations. Encapsulation is probably better at reducing cuttings dispersion than inhibiting hydration of the near wellbore shale. We can describe PHPA more accurately as a high molecular weight copolymer of acrylamide and acrylic acid. The amide and carboxylate functional groups adsorb very firmly onto the cutting surface. This action will coat them with a layer of viscous polymer that helps to slow the flow of filtrate into the formation pores.
Water Permeation Rate & Shale Inhibitor
Reduction of Water Permeation Rate into Shale by increasing the viscosity of the aqueous phase slows the hydration process. According to Darcy’s Law, the permeation flow rate is inversely proportional to the viscosity. Accordingly, the flow rate of a salt-saturated filtrate into a porous solid would be half the freshwater flow rate. This is because the viscosity of saturated sodium chloride solution is twice that of water.
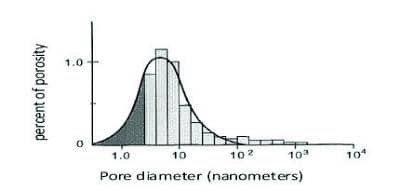
Solutions of only relatively low molecular weight materials that work as shale inhibitors (such as salts, polyglycerols, methyl glucoside, and polyalkylene glycols) can flow through shale pores because the pore size is tiny. The graph below shows that most of the pores in a typical shale are less than 10 nanometers in diameter. For a typical PHPA polymer with a molecular weight over one million, a dissolved PHPA molecule can be as long as about 1,000 nm (or 1 micron). Therefore, it cannot flow through the pore network.
How The Shale Inhibitor Called Polyalkylene Glycols (PAGS) Work
Replacement of Inter-Layer Water using polyalkylene glycols (PAGs) as a shale inhibitor, which are typically low (around 1,000) molecular weight copolymers of ethylene oxide and propylene oxide. Sometimes, we may use ethylene oxide independently, as in polyethylene glycol, or an ethoxylated alcohol such as butanol ethoxylate. The ethylene oxide type glycols must be used with potassium for good effect.
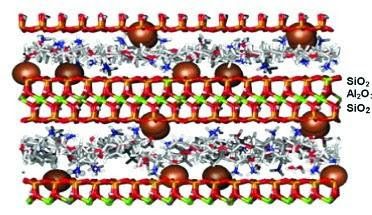
- Potassium ions are shown as spheres occupying the cation exchange sites.
- The polyalkylene glycol molecules displace water, filling the gap between the sheets.
Ethylene oxide and propylene oxide copolymers can be effective without using potassium chloride. The ether-links (-C—O—C-) in PAGs adsorb onto montmorillonite sheet surfaces more strongly than water, so they can infiltrate the gap, displace water, and stop further hydration. Recently, new PAG molecules with amine “anchor groups” at each end have been developed. These molecules are similar to the one shown below:
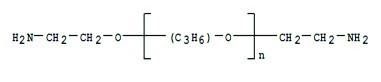
The Effect Of Osmotic Transfer of Water On Shale Inhibition
Reduction or Reversal of Osmotic Transfer of Water into Shale requires a Semi-Permeable Membrane that allows water molecules, but not solutes such as salts, to diffuse through the tiny pores in the membrane. The membrane also restricts the flow of liquid water. Shale can act like a semi-permeable membrane, but pore-plugging additives can improve its efficiency.
Osmosis is mainly an issue with Water Based Mud, and it has increased in importance as the efficiency of commercial additives has improved over the years. The more reduction of the shale permeability and the more inhibition of the shale hydration, the more likely it is that the shale approaches the condition of a semi-permeable membrane. Under these conditions, using a reduced Water Activity (Aw) brine phase in the Drilling Mud becomes more critical to avoid the osmotic build-up of high pore pressure in the shale, which can lead to tight holes and/or cavings.
Water Activity
Water Activity is related to water vapor pressure. Water vapor is composed of individual molecules of water. In liquid water, individual molecules move around (diffuse) in equilibrium with larger groups of water molecules held transiently together by hydrogen bonding. The concentration of single water molecules is a function of temperature. For example, at boiling point, several individual water molecules are moving about rapidly enough to create a vapor pressure of 1 atmosphere.
Water vapor pressure is also a function of the concentration of dissolved material in the water. When drilling fluid diffuses into shale pores, clay surfaces capture water molecules. This action will restore the hydration by dissolved ions in the pore water. The Water Activity (Aw) coefficient, representing the difference in vapor pressure between pure water and brine saturated with sodium chloride (25% by weight NaCl), is a factor of 0.75.
If the water activity in the Drilling Fluid is high, there will be a plentiful supply of water vapor molecules diffusing from the mud into the shale. This will raise the pore pressure, which, together with continued diffusion, will transport water of hydration further into the shale. If the water activity in the drilling mud is lower than that of the shale, the drilling fluid will capture the water molecules that would have diffused into the shale. This will cause a net flow of water into the mud, reducing the shale’s hydration.h reduces the hydration of the shale. This diffusion and capture of water molecules is a form of osmosis.
“Leaky Semi-permeable Membrane” Effect
Shale compaction will lead to squeezing out inter-particle water, leaving less mobile ions like sodium, magnesium, calcium, and chloride in the shale pores. This increased pore water salinity and decreased shale water activity, reducing water vapor pressure.
In addition, the clay particles became compressed tightly by the overburden pressure over time, and illite and montmorillonite particles became compressed as inter-layer water escaped. This puts a lot of stored energy into the compressed shale, so, given the chance, the shale wants to rehydrate and expand. So, the compressed shale also results in reduced water activity, which adds to the contribution of the concentrated salts in the pore water. As mentioned earlier, we can improve the efficiency of the semipermeable shale membrane by using pore-plugging additives.
Inhibition Mechanism Of Plugging of Shale Pores
Through the illustration presented, it becomes apparent that plugging shale pores will cause a reduction of pore pressure penetration. Using small organic colloids or clouded-out PAG droplets to block pores near the surface of the wellbore can substantially decrease shale permeability. This reduction in permeability prevents the hydrating water and wellbore pressure from penetrating deep into the shale, leaving the hydrostatic pressure of the drilling fluid to act upon the wellbore’s surface and support the near-wellbore shale.
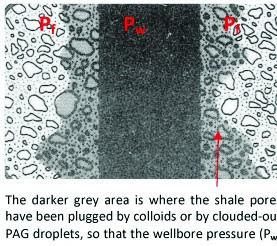
Besides organic colloids and polalkylene glycols, inhibitive drilling mud based on the sodium silicate shale inhibitor is thought to cause near-wellbore pore plugging in shale. The alkaline silicate solution reacts with the clay in the shale and the ions in the pore water. The subsequent drop in pH causes silica to precipitate, and calcium and magnesium dissolved in the pore water cause insoluble silicate salts to precipitate. Both of these colloidal precipitates are effective pore-pluggers.
Shale Micro Fractures Sealing Solution
Sealing of Shale Micro-Fractures is necessary when shales are brittle and contain many minor fractures and micro-cracks. The diagram below shows that the mud pressure can leak into the cracks, leading to little or no differential pressure (ΔP) supporting the near-wellbore shale. This can result in spalling, where pieces of shale break off and fall into the wellbore, and hole gauge washouts.
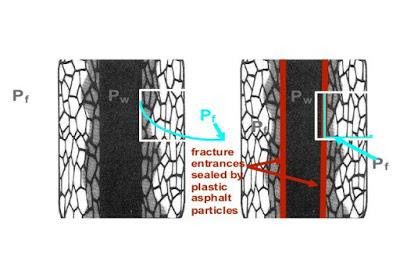
Some materials used as shale inhibitors, such as blown asphalt or gilsonite, may be brittle, especially at surface temperatures. These materials can be ground to a size similar to that of the microcracks in shale. When added to a drilling fluid, the powdered particles become lodged in the openings of fractures in the shale. Upon exposure to elevated downhole temperatures, these particles soften and come together, sealing the openings to the cracks. This contains the pressure from the wellbore within the near wellbore shale, preventing spalling and caving of shale into the wellbore, helping to maintain the integrity of the borehole wall.
How Chemical Reactions Can Cause Shale Inhibition
Earlier, we explored how is the production process of inter-particle cement through reactions with clays in shale. The process involves the alkaline filtrate obtained from sodium silicate fluids reacting with the clay in the shale. The high alkalinity can dissolve some silica from the silica layers of the clay, and the alumina layers can dissolve transiently to an extent that leads to the production of sodium aluminate in the solution. These reactions decrease the alkalinity of the invaded filtrate, resulting in silica precipitation. The aluminate and silicates also combine to form amorphous alumino-silicates, which eventually settle out.
Besides the pore-plugging effects of these precipitates noted earlier, they act as intergranular cement, which causes the shale to harden and gain strength. By these processes, sodium silicate fluids have occasionally provided the levels of wellbore stability only obtained previously by using oil-based mud. However, some drawbacks seen with silicate systems have included cuttings accretion onto BHAs, poor lubricity, interference with MWD tools, and difficulty obtaining low fluid loss.
It is worth mentioning that many companies use high-lime fluids in shale drilling. The objective here is that the lime in the filtrate reacts with clay particles in the shale in ways similar to Portland cement or lime mortars to produce a calcium silicate cementitious precipitate. The use of lime fluids for shale drilling has declined with the development of more efficient inhibitive fluids and additives.
Scientific Papers
- Design and Development of Quaternary Amine Compounds: Shale Inhibition With Improved Environmental Profile
- Mechanism of Shale Inhibition by Polyols in Water-Based Drilling Fluids
- Guidelines for Shale Inhibition During Openhole Gravel Packing With Water-Based Fluids
- Enhancing the rheological properties and shale inhibition behavior of water-based mud using nano-silica, multi-walled carbon nanotube, and graphene nanoplatelet